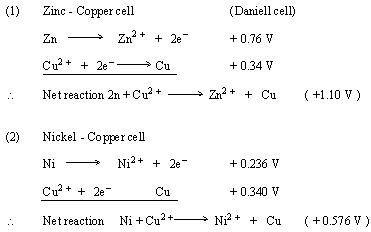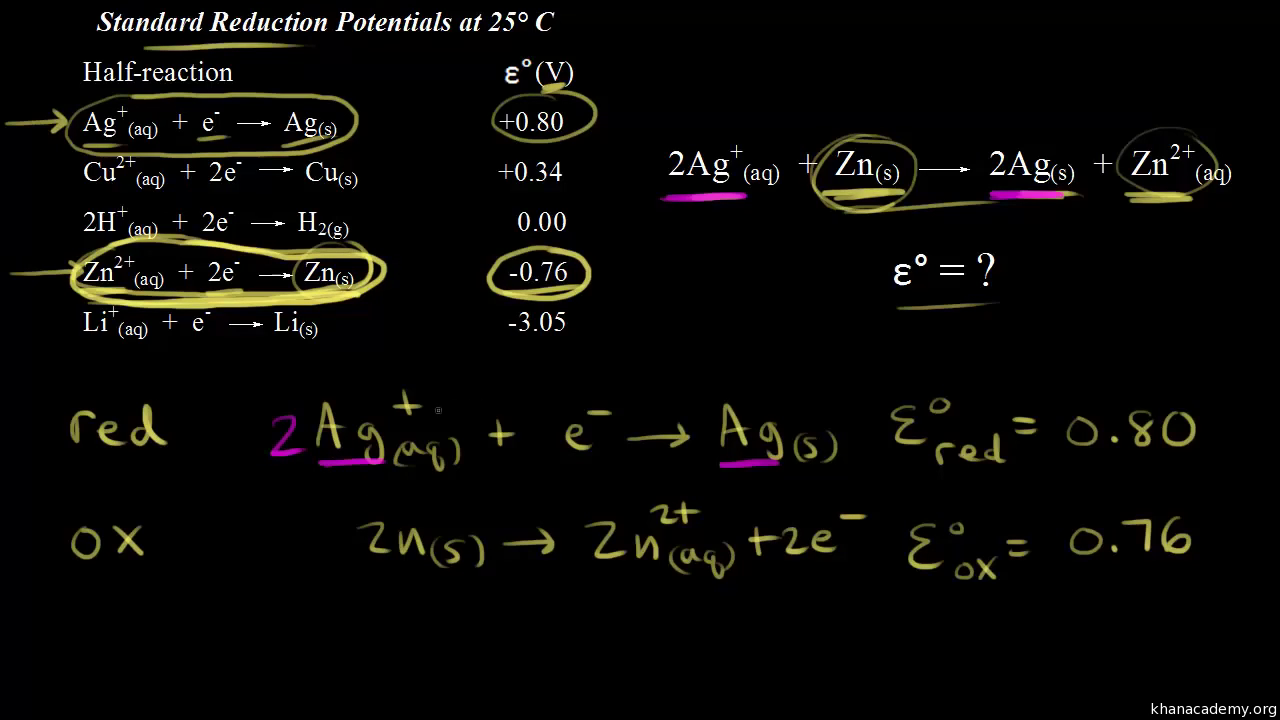
Electrode Potentials and Electrochemical Cells - Electrode Potentials (A-Level Chemistry) - Study Mind

Calculate the standard cell potentials of galvanic cell in whiCHM the following reactions take p... - YouTube

Calculate the standard cell potential in (V) of the cell in which following reaction takes place: Fe^2 + (aq) + Ag^ + (aq) → Fe^3 + (aq) + Ag(s) Given that E^oAg^ + /
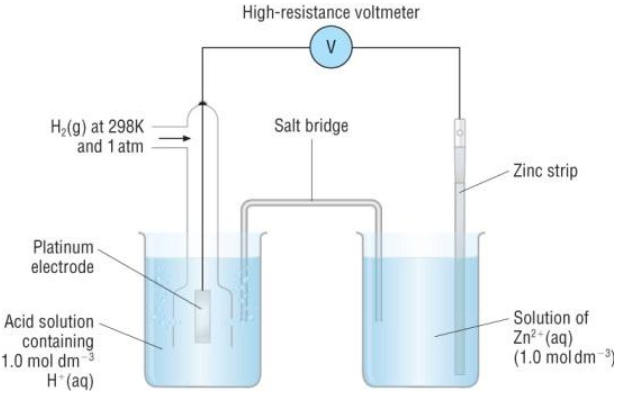
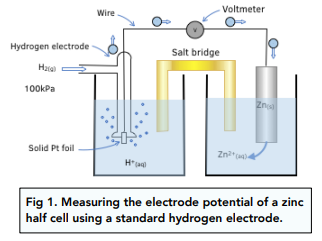
How to calculate the potential of zinc electrode capacity when in contact with 0.1M zinc sulphate solution in reference to hydrogen electrode when given the standard cell potential of Zn2 + /

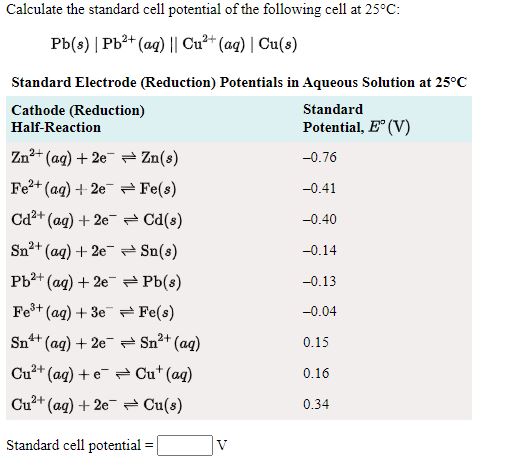
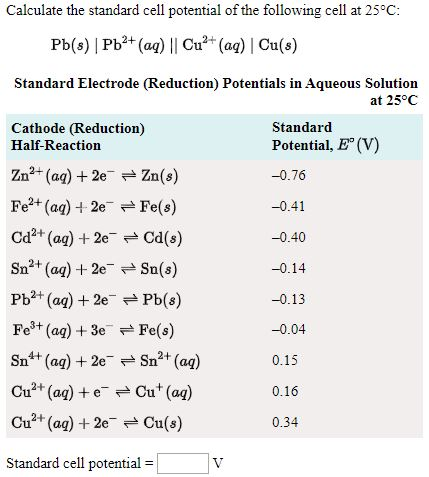
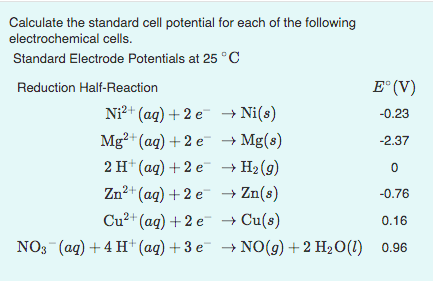
SOLVED: Calculate the standard cell potential of the following cell at 25'C: Pb(s) Pb2+ (aq) || Cu? + (aq) Cu(s) Standard Electrode (Reduction) Potentials in Aqueous Solution at 258C Cathode (Reduction) Half-Reaction

Standard Cell Potential: Calculations, Electron Flow & Feasibility (5.4.2) | CIE A Level Chemistry Revision Notes 2022 | Save My Exams
![PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/56964684a624c5af38c7e62256db3faa4c542d88/19-Table2-1.png)
PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar